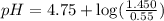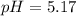Chemistry, 04.04.2020 11:34 anniekwilbourne
Enter your answer in the provided box. Calculate the pH of 1.00 L of a buffer that is 1.00 M in acetic acid and 1.00 M in sodium acetate after the addition of 0.450 mole of NaOH.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
Enter your answer in the provided box. Calculate the pH of 1.00 L of a buffer that is 1.00 M in acet...
Questions


Mathematics, 03.08.2019 09:30



English, 03.08.2019 09:30

History, 03.08.2019 09:30

Mathematics, 03.08.2019 09:30






Social Studies, 03.08.2019 09:30

Mathematics, 03.08.2019 09:30



History, 03.08.2019 09:30



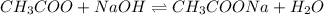
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0582/2902/e961a.png)
![pH=pK_a+\log \frac{[CH_3COONa]}{[CH_3COOH]}](/tpl/images/0582/2902/023d4.png)