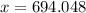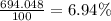Chemistry, 04.04.2020 11:42 PrincessIndia
Lithium has two stable isotopes with masses of 6.01512 amu and 7.01600 amu. The average molar mass of Li is 6.941 amu. What is the percent abundance of each isotope? Show all calculations and report to the correct number of sig figs.

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 02:30
what is your question? collegechemistry 5+3 pts in november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
You know the right answer?
Lithium has two stable isotopes with masses of 6.01512 amu and 7.01600 amu. The average molar mass o...
Questions

English, 09.07.2019 09:30

Mathematics, 09.07.2019 09:30

Chemistry, 09.07.2019 09:30

Arts, 09.07.2019 09:30


Mathematics, 09.07.2019 09:30


Mathematics, 09.07.2019 09:30


Mathematics, 09.07.2019 09:30

History, 09.07.2019 09:30

Mathematics, 09.07.2019 09:30

Chemistry, 09.07.2019 09:30

Mathematics, 09.07.2019 09:30



Biology, 09.07.2019 09:30

History, 09.07.2019 09:30


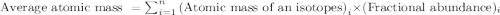 .....(1)
.....(1)
![6.941=[(6.01512\times x)+(7.01600\times (100-x))]](/tpl/images/0582/2997/75f4c.png)