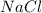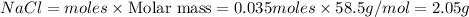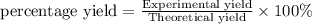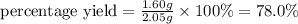Chemistry, 04.04.2020 12:41 shawnaelvaughns
Aqueous hydrochloric acid reacts with solid sodium hydroxide to produce aqueous sodium chloride and liquid water . If of sodium chloride is produced from the reaction of of hydrochloric acid and of sodium hydroxide, calculate the percent yield of sodium chloride. Round your answer to significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3
You know the right answer?
Aqueous hydrochloric acid reacts with solid sodium hydroxide to produce aqueous sodium chloride and...
Questions


Social Studies, 31.07.2019 07:00

Biology, 31.07.2019 07:00

History, 31.07.2019 07:00


Biology, 31.07.2019 07:00

Mathematics, 31.07.2019 07:00

History, 31.07.2019 07:00


Spanish, 31.07.2019 07:00

History, 31.07.2019 07:00


Biology, 31.07.2019 07:00

Biology, 31.07.2019 07:00

History, 31.07.2019 07:00





History, 31.07.2019 07:00

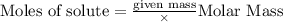
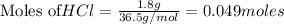
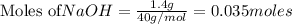
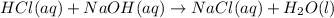
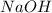 require = 1 mole of
require = 1 mole of 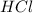
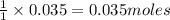 of
of