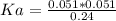Calculate the Ka for the following acid. Determine if it is a strong or weak acid. HClO2(aq) dissolves in aqueous solution to form H+(aq) and ClO2−(aq). At equilibrium, the concentrations of each of the species are as follows: [HClO2]=0.24M [H+]=0.051M [ClO2−]=0.051M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
Calculate the Ka for the following acid. Determine if it is a strong or weak acid. HClO2(aq) dissolv...
Questions



Mathematics, 05.05.2020 18:12



Physics, 05.05.2020 18:12


Health, 05.05.2020 18:12


History, 05.05.2020 18:13

History, 05.05.2020 18:13

Mathematics, 05.05.2020 18:13


History, 05.05.2020 18:13




Social Studies, 05.05.2020 18:13



![[HClO_2]=0.24M](/tpl/images/0582/3449/64bd9.png)
![[H^+]=0.051M](/tpl/images/0582/3449/e1645.png)
![[ClO_2^-]=0.051M](/tpl/images/0582/3449/4a4bd.png)
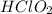 is
is
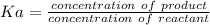
![= \frac{[H^+][ClO_2^-]}{[HClO_2]}](/tpl/images/0582/3449/87292.png)