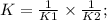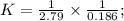Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1


Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

Chemistry, 23.06.2019 06:40
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1
You know the right answer?
Two reactions and their equilibrium constants are given. A + 2 B − ⇀ ↽ − 2 C K 1 = 2.79 2 C − ⇀ ↽ −...
Questions




English, 01.09.2019 05:20

Mathematics, 01.09.2019 05:20


History, 01.09.2019 05:20




Social Studies, 01.09.2019 05:20


Social Studies, 01.09.2019 05:20

Physics, 01.09.2019 05:20

Social Studies, 01.09.2019 05:20



History, 01.09.2019 05:20


Social Studies, 01.09.2019 05:20