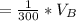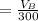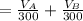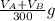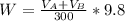Chemistry, 04.04.2020 14:09 vladsmolin7781
The solubility of aspirin in water is 1 g per 300 mL at 25 degrees celsius. Assuming that your crystallization and washing with water were done at this temperature, what weight of aspirin did you lose in the filtrate and washings? How much was your percent yield lowered by this loss?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

You know the right answer?
The solubility of aspirin in water is 1 g per 300 mL at 25 degrees celsius. Assuming that your cryst...
Questions

English, 10.11.2020 17:40

Mathematics, 10.11.2020 17:40

Biology, 10.11.2020 17:40

Mathematics, 10.11.2020 17:40

Social Studies, 10.11.2020 17:40

English, 10.11.2020 17:40


Mathematics, 10.11.2020 17:40

English, 10.11.2020 17:40



Chemistry, 10.11.2020 17:40

English, 10.11.2020 17:40

Mathematics, 10.11.2020 17:40


Health, 10.11.2020 17:40


English, 10.11.2020 17:40


Mathematics, 10.11.2020 17:40

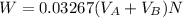
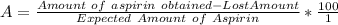
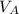
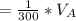
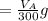
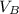 to wash the crystallized aspirin then the lost during washing would be
to wash the crystallized aspirin then the lost during washing would be