Chemistry, 04.04.2020 14:34 wirchakethan23
The reform reaction between steam and gaseous methane () produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. Synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen. Suppose a chemical engineer studying a new catalyst for the reform reaction finds that liters per second of methane are consumed when the reaction is run at and . Calculate the rate at which dihydrogen is being produced. Give your answer in kilograms per second. Round your answer to significant digits..

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

You know the right answer?
The reform reaction between steam and gaseous methane () produces "synthesis gas," a mixture of carb...
Questions



Mathematics, 02.03.2021 02:40





Mathematics, 02.03.2021 02:40



Mathematics, 02.03.2021 02:40

Biology, 02.03.2021 02:40

Mathematics, 02.03.2021 02:40







Biology, 02.03.2021 02:40

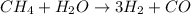
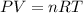
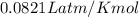
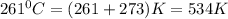
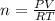
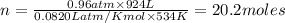
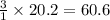 moles of hydrogen
moles of hydrogen