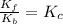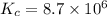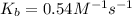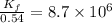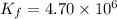Chemistry, 04.04.2020 14:31 eburnhisel2023
At 700 K the equilibrium constant KC for the reaction between NO(g) and O2(g) forming NO2(g) is 8.7 × 106. The rate constant for the reverse reaction at this temperature is 0.54 M–1s–1. What is the value of the rate constant for the forward reaction at 700 K?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1


Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3
You know the right answer?
At 700 K the equilibrium constant KC for the reaction between NO(g) and O2(g) forming NO2(g) is 8.7...
Questions



Mathematics, 16.10.2020 04:01



Mathematics, 16.10.2020 04:01

History, 16.10.2020 04:01


Mathematics, 16.10.2020 04:01


Mathematics, 16.10.2020 04:01



Mathematics, 16.10.2020 04:01

History, 16.10.2020 04:01


History, 16.10.2020 04:01

Mathematics, 16.10.2020 04:01

Physics, 16.10.2020 04:01

Medicine, 16.10.2020 04:01

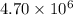
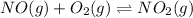
![K_c=\frac{[NO_2]}{[NO][O_2]}](/tpl/images/0582/5120/67e8f.png)
![R_f=K_f[NO][O_2]](/tpl/images/0582/5120/9cd84.png)
![R_b=K_b[NO_2]](/tpl/images/0582/5120/d91b1.png)
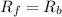
![K_f[NO][O_2]=K_b[NO_2]](/tpl/images/0582/5120/7432e.png)
![\frac{K_f}{K_b}=\frac{[NO_2]}{[NO][O_2]}](/tpl/images/0582/5120/419f2.png)