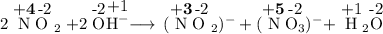Which type of reaction occurs in the following equation?
2NO2(g) + 2OH"(aq) —— NO2(aq) + NO, (...

Chemistry, 04.04.2020 18:10 kimjooin02
Which type of reaction occurs in the following equation?
2NO2(g) + 2OH"(aq) —— NO2(aq) + NO, (aq) +H2O(1)
a combination redox reaction
a displacement redox reaction
a synthetic redox reaction
a disproportionation redox reaction

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
You know the right answer?
Questions


Social Studies, 17.04.2021 09:40

Mathematics, 17.04.2021 09:40


History, 17.04.2021 09:40




English, 17.04.2021 09:40




Business, 17.04.2021 09:50



Social Studies, 17.04.2021 09:50

Computers and Technology, 17.04.2021 09:50


Social Studies, 17.04.2021 09:50