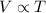Chemistry, 04.04.2020 18:25 ravenjade2395
Jorge inflates a beach ball to a volume of 4L in his air-conditioned house where the temperature is 18℃. That afternoon he takes the beach ball to the beach with some friends. The temperature at the beach is 32℃, and the air pressure at the beach is the same as it was at Jorge's house. What will happen to Jorge's beach ball when he is at the beach? Choose the claim that best answers the question: * 1 point The beach ball will get smaller at the beach because the molecules are moving slower. The beach ball will stay the same size at the beach because the pressure is constant. The beach ball will get larger at the beach because the molecules are moving faster. The beach ball will get larger at the beach because the pressure will cause it to expand. The beach ball will get smaller at the beach because the molecules will collide less often.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

You know the right answer?
Jorge inflates a beach ball to a volume of 4L in his air-conditioned house where the temperature is...
Questions





History, 29.05.2021 15:30




English, 29.05.2021 15:40

Mathematics, 29.05.2021 15:40





Business, 29.05.2021 15:40

Mathematics, 29.05.2021 15:40


Chemistry, 29.05.2021 15:40

Chemistry, 29.05.2021 15:40

Social Studies, 29.05.2021 15:40