Chemistry, 04.04.2020 21:02 kevinhill185
A chemist reacts magnesium with hydrochloric acid and collects 57.48 mL of the hydrogen gas that is produced by water displacement. If the lab temperature is 24 C and the atmospheric pressure is 742.1 mm Hg, how many grams of hydrogen are produced? Water vapor pressure is 22.4 mm Hg at 24 C.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
A chemist reacts magnesium with hydrochloric acid and collects 57.48 mL of the hydrogen gas that is...
Questions


Mathematics, 08.01.2020 02:31





Mathematics, 08.01.2020 02:31




Chemistry, 08.01.2020 02:31


History, 08.01.2020 02:31

Social Studies, 08.01.2020 02:31






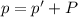
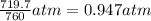
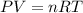 ( Ideal gas equation)
( Ideal gas equation)