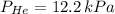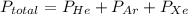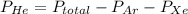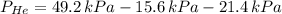Chemistry, 04.04.2020 21:30 maisonsuperman5321
A mixture of helium, argon, and xenon gases are present in a container. What is the partial pressure of helium if the total pressure is 49.2 kPa and the partial pressure of Ar is 15.6 kPa and 21,4 kPa for Xe?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3


Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
A mixture of helium, argon, and xenon gases are present in a container. What is the partial pressure...
Questions




Mathematics, 15.07.2019 21:30


Mathematics, 15.07.2019 21:30

Computers and Technology, 15.07.2019 21:30

Biology, 15.07.2019 21:30












Computers and Technology, 15.07.2019 21:30