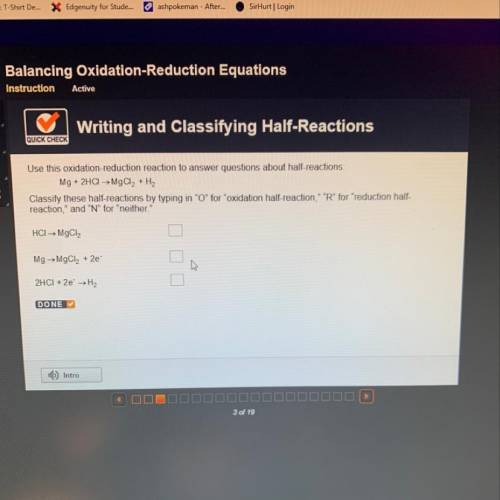
Use this oxidation-reduction reaction to answer questions about half-reactions:
Mg + 2HCl → Mg...

Chemistry, 05.04.2020 04:59 Haleysaraya1
Use this oxidation-reduction reaction to answer questions about half-reactions:
Mg + 2HCl → MgCl2 + H2
Classify these half-reactions by typing in "O" for "oxidation half-reaction," "R" for "reduction half-
reaction," and "N" for "neither."
HCI→ MgCl2
Mg → MgCl2 + 2e
2HCI + 2e →H2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Questions

Mathematics, 13.02.2020 08:23


Mathematics, 13.02.2020 08:24


Mathematics, 13.02.2020 08:25

History, 13.02.2020 08:25

Mathematics, 13.02.2020 08:25


Mathematics, 13.02.2020 08:25

Mathematics, 13.02.2020 08:25

English, 13.02.2020 08:25

History, 13.02.2020 08:25


History, 13.02.2020 08:25

Mathematics, 13.02.2020 08:25


Mathematics, 13.02.2020 08:25


Mathematics, 13.02.2020 08:26




