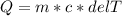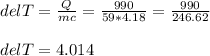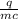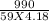JC and D
If 990.J of heat is absorbed by a 59 g sample of water at 21.0 °C, what
will the final temperature of this sample be after absorbing this energy
The specific heat of water is 4.18 J/9g °C). *
25 degrees Celsius
O
35 degrees Celsius
o
2.5 degrees Celsius

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3


Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
JC and D
If 990.J of heat is absorbed by a 59 g sample of water at 21.0 °C, what
will th...
If 990.J of heat is absorbed by a 59 g sample of water at 21.0 °C, what
will th...
Questions

History, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Social Studies, 11.09.2020 07:01

History, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Physics, 11.09.2020 07:01

Social Studies, 11.09.2020 07:01