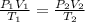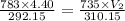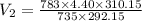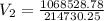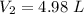During laparoscopic surgery, carbon dioxide gas is used to expand the abdomen to help create a larger working space.
If 4.40 L of CO2 gas at 19 ∘C at 783 mmHg is used, what is the final volume, in liters, of the gas at 37 ∘C and a pressure of 735 mmHg, if the amount of CO2 does not change?
Express your answer with the appropriate units.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
During laparoscopic surgery, carbon dioxide gas is used to expand the abdomen to help create a large...
Questions

Health, 16.12.2020 17:50

Mathematics, 16.12.2020 17:50




English, 16.12.2020 17:50






Mathematics, 16.12.2020 17:50



Mathematics, 16.12.2020 17:50


Social Studies, 16.12.2020 17:50

Mathematics, 16.12.2020 17:50

English, 16.12.2020 17:50

Physics, 16.12.2020 17:50