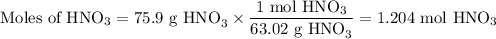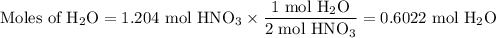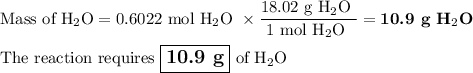Not understanding on how stoichiometry works not sure which side to start on. ex: How many grams of water are required to form 75.9 g of HNO3? Assume that there is excess NO2 present. The molar masses are as follows: H2O = 18.02 g/mol, HNO3 = 63.02 g/mol.
3 NO2(g) + H2O(l) → 2 HNO3(aq) + NO(g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1


Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
Not understanding on how stoichiometry works not sure which side to start on. ex: How many grams of...
Questions









Mathematics, 27.05.2021 19:20

Biology, 27.05.2021 19:20

Mathematics, 27.05.2021 19:20

Mathematics, 27.05.2021 19:20

Mathematics, 27.05.2021 19:20

Mathematics, 27.05.2021 19:20



Mathematics, 27.05.2021 19:20


Mathematics, 27.05.2021 19:20

History, 27.05.2021 19:20