
Chemistry, 06.04.2020 01:02 winterblackburn78
According to the following equation, Calculate the percentage yield if 550.0 g of toluene (C7H8 )added to an excess of nitric acid (HNO3) provides 305 g of the p-nitrotoluene (C7H7NO2 ) product in a lab experiment.
___C7H8 + ___HNO3 ___C7H7NO2 + ___H2O

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
According to the following equation, Calculate the percentage yield if 550.0 g of toluene (C7H8 )add...
Questions

English, 12.12.2020 15:50

Biology, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50



German, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50

History, 12.12.2020 15:50




English, 12.12.2020 15:50


Chemistry, 12.12.2020 15:50

Spanish, 12.12.2020 15:50





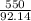
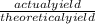 x 100
x 100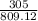 x 100
x 100

