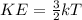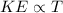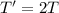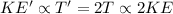Question 1
According to the kinetic molecular theory, as the absolute temperature of a gas is...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1

You know the right answer?
Questions






English, 23.07.2019 12:30


Mathematics, 23.07.2019 12:30

History, 23.07.2019 12:30


History, 23.07.2019 12:30

History, 23.07.2019 12:30

Mathematics, 23.07.2019 12:30

Chemistry, 23.07.2019 12:30

Biology, 23.07.2019 12:30





Mathematics, 23.07.2019 12:30