
Chemistry, 06.04.2020 11:30 justice808
4. Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide. Assume all gases are at the same conditions of temperature and pressure.
2N2(g) + 3O2(g) 2N2O3(g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2



Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
You know the right answer?
4. Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide...
Questions






Mathematics, 09.09.2021 18:30

Mathematics, 09.09.2021 18:30

Chemistry, 09.09.2021 18:30

Mathematics, 09.09.2021 18:30







History, 09.09.2021 18:30



Health, 09.09.2021 18:30

Mathematics, 09.09.2021 18:30

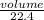
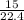 = 0.67moles
= 0.67moles 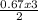 = 1.01 moles of O₂
= 1.01 moles of O₂

