
Chemistry, 06.04.2020 18:02 wilkoASK2919
For the reaction 2A(g) â B(g), the equilibrium constant is Kp = 0.76. A reaction mixture initially contains 4.0 atm of gas (PA = 2.0 atm and PB = 2.0 atm).
Which statement is true of the reaction mixture?
(a) The reaction mixture will proceed toward products.
(b) The reaction mixture is at equilibrium.
(c) The reaction mixture will proceed toward reactants
(d) It is not possible to determine from the information given the future direction of the reaction mixture

Answers: 1
Another question on Chemistry




Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
For the reaction 2A(g) â B(g), the equilibrium constant is Kp = 0.76. A reaction mixture initially c...
Questions

History, 30.11.2019 23:31

Mathematics, 30.11.2019 23:31

Mathematics, 30.11.2019 23:31


Mathematics, 30.11.2019 23:31


Mathematics, 30.11.2019 23:31



Social Studies, 30.11.2019 23:31

Spanish, 30.11.2019 23:31



Mathematics, 30.11.2019 23:31


Mathematics, 30.11.2019 23:31




Business, 30.11.2019 23:31

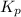
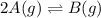
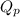 is written as:
is written as: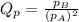
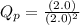
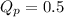
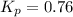
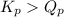 , the reaction will shift towards the right i.e. towards the product side.
, the reaction will shift towards the right i.e. towards the product side.

