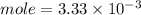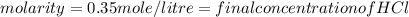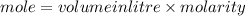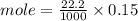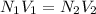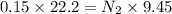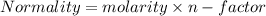Chemistry, 06.04.2020 18:02 hntnhtthnyt
Two burettes are set up. The first contains 0.15M NaOH and the second contains an HCl solution of unknown concentration. HCl is dispensed into the reaction flask and Phenolphthalein is added as an indicator. At the end of the titration, 22.2ml of NaOH and 9.45ml HCl were used.
Required:
A. Write a balanced equation for the Acid-Base reaction.
B. What is the total volume of NaOH used in the titration?
C. How many moles of NaOH was used?
D. What is the total volume of HCl used in the titration?
E. What is the final concentration of the HCl solution?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1


Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

You know the right answer?
Two burettes are set up. The first contains 0.15M NaOH and the second contains an HCl solution of un...
Questions

Biology, 15.07.2019 16:00


Mathematics, 15.07.2019 16:00


History, 15.07.2019 16:00


Mathematics, 15.07.2019 16:00


Social Studies, 15.07.2019 16:00


Biology, 15.07.2019 16:00

Mathematics, 15.07.2019 16:00

Chemistry, 15.07.2019 16:00







Mathematics, 15.07.2019 16:00