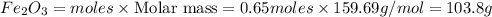Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1
You know the right answer?
An iron nail rusts when exposed to oxygen. According to the following reaction, how many grams of ir...
Questions


Physics, 01.07.2019 03:30

Social Studies, 01.07.2019 03:30

Biology, 01.07.2019 03:30



French, 01.07.2019 03:30


Mathematics, 01.07.2019 03:30


Health, 01.07.2019 03:30

Mathematics, 01.07.2019 03:30

Mathematics, 01.07.2019 03:30

History, 01.07.2019 03:30


History, 01.07.2019 03:30



Mathematics, 01.07.2019 03:30

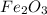 , 0.65 moles of
, 0.65 moles of 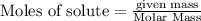
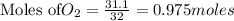
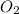 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and 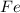 is the excess reagent.
is the excess reagent.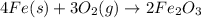
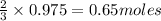 of
of