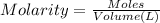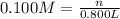Chemistry, 06.04.2020 18:54 devinmoore4664
A chemist must prepare of 800.0 ml potassium hydroxide solution with a pH of 13.00 at 25°.
She will do this in three steps:
Fill a 800 ml volumetric flask about halfway with distilled water.
Weigh out a small amount of solid potassium hydroxide and add it to the flask.
Fill the flask to the mark with distilled water.
Calculate the mass of potassium hydroxide that the chemist must weigh out in the second step. Round your answer to significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
A chemist must prepare of 800.0 ml potassium hydroxide solution with a pH of 13.00 at 25°.
Questions


Mathematics, 11.06.2020 18:57

Mathematics, 11.06.2020 18:57

English, 11.06.2020 18:57

Mathematics, 11.06.2020 18:57







Mathematics, 11.06.2020 18:57








![pOH=-\log[OH^-]](/tpl/images/0584/6258/fe336.png)
![1.00=-\log[OH^-]](/tpl/images/0584/6258/6b8f7.png)
![[OH^-]=10^{-1.00} M=0.100 M](/tpl/images/0584/6258/a9d8c.png)
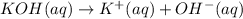
![[KOH]=[OH^-]=[K^+]=0.100 M](/tpl/images/0584/6258/fca0f.png)