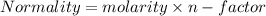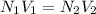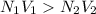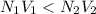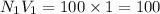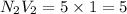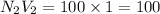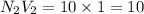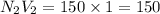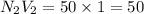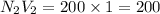Chemistry, 07.04.2020 01:23 LTLICKME7437
A volume of 100 mL of 1.00 M HCl solution is titrated with 1.00 M NaOH solution. You added the following quantities of 1.00 M NaOH to the reaction flask. Classify the following conditions based on whether they are before the equivalence point, at the equivalence point, or after the equivalence point.
a. 5.00 mL of 1.00 M NaOH
b. 100mL of 1.00 M NaOH
c. 10.0 mL of 1.00 M NaOH
d. 150 mL of 1.00 M NaOH
e. 50.0 mL of 1.00 M NaOH
f. 200 mL of 1.00 M NaOH

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1
You know the right answer?
A volume of 100 mL of 1.00 M HCl solution is titrated with 1.00 M NaOH solution. You added the follo...
Questions

Biology, 23.03.2020 22:24

Biology, 23.03.2020 22:24


Social Studies, 23.03.2020 22:24


Mathematics, 23.03.2020 22:24

Computers and Technology, 23.03.2020 22:24

English, 23.03.2020 22:24

History, 23.03.2020 22:24

Physics, 23.03.2020 22:24




History, 23.03.2020 22:24

History, 23.03.2020 22:24

Mathematics, 23.03.2020 22:24

Mathematics, 23.03.2020 22:24


Chemistry, 23.03.2020 22:24