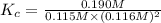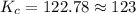Consider the following reaction: CO(g)+2H2(g) <--> CH3OH(g).
An equilibrium mixture of t...

Chemistry, 07.04.2020 02:36 maddysmall32
Consider the following reaction: CO(g)+2H2(g) <--> CH3OH(g).
An equilibrium mixture of this reaction at a certain temperature was found to have
[CO]=0.115M, [H2]=0.116M, and [CH3OH]=0.190M. What is the value of the equilibrium constant (Kc) at this temperature?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2


Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
Questions



Mathematics, 07.05.2020 07:58

Computers and Technology, 07.05.2020 07:58



Chemistry, 07.05.2020 07:58

Mathematics, 07.05.2020 07:58

Mathematics, 07.05.2020 07:58

English, 07.05.2020 07:58



Biology, 07.05.2020 07:58

Physics, 07.05.2020 07:58





English, 07.05.2020 07:58

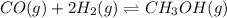
![[CO]=0.115 M,[H_2]=0.116 M](/tpl/images/0585/7114/6fdc2.png)
![[CH_3OH]=0.190 M](/tpl/images/0585/7114/98ee3.png)
![K_c=\frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0585/7114/4cf94.png)