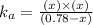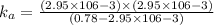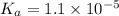Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1


Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
The pH of a 0.78M solution of 4-pyridinecarboxylic acid HC6H4NO2is measured to be 2.53.Calculate the...
Questions


Social Studies, 25.11.2020 17:10

Mathematics, 25.11.2020 17:10










English, 25.11.2020 17:10



English, 25.11.2020 17:10




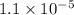
![pH=-\log [H^+]](/tpl/images/0585/8119/37e81.png)
![2.53=-\log [H^+]](/tpl/images/0585/8119/2972b.png)
![[H^+]=2.95\times 106{-3}M](/tpl/images/0585/8119/c8da4.png)
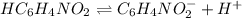
![k_a=\frac{[C_6H_4NO_2^-][H^+]}{[C_6H_4NO_2]}](/tpl/images/0585/8119/d42d8.png)
![[H^+]=[C_6H_4NO_2^-]=x](/tpl/images/0585/8119/beac4.png)