
Chemistry, 07.04.2020 03:19 izzybella18ozgsrg
The molar mass of Allura red is 496 g/mol. The stock solution contains 93.84 mg/L Allura red. ac. To create 100.00 mL of a 1.89 x 10-6 M solution, you would need this many mL of the stock solution.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
The molar mass of Allura red is 496 g/mol. The stock solution contains 93.84 mg/L Allura red. ac. To...
Questions




Mathematics, 29.01.2020 02:44

History, 29.01.2020 02:44

Mathematics, 29.01.2020 02:44


Health, 29.01.2020 02:44

Health, 29.01.2020 02:44






Computers and Technology, 29.01.2020 02:44





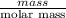
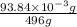
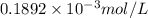
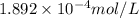
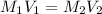
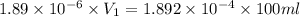
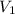 = 1.0 ml
= 1.0 ml

