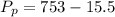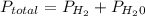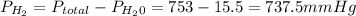Chemistry, 07.04.2020 03:19 blueyish6422
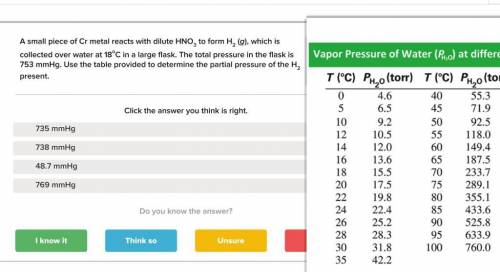
A small piece of Cr metal reacts with dilute HNO3 to form H2 (g), which is collected over water at 18 C in a large flask. The total pressure in the flask is 753 mmHg.
Determine the partial pressure of the H2 present.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

You know the right answer?
A small piece of Cr metal reacts with dilute HNO3 to form H2 (g), which is collected over water at 1...
Questions









History, 21.11.2019 20:31



Biology, 21.11.2019 20:31

Mathematics, 21.11.2019 20:31




Biology, 21.11.2019 20:31

Biology, 21.11.2019 20:31


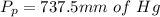
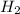 is mathematically represented as
is mathematically represented as 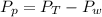
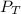 is the total pressure of water with a value of 15.5 mm of Hg
is the total pressure of water with a value of 15.5 mm of Hg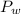 is the partial pressure of water with a value 753 mm of Hg
is the partial pressure of water with a value 753 mm of Hg