
Chemistry, 07.04.2020 04:13 crarylolmeow
HBrO(aq) + H2O(l) ⇄ H3O+(aq) + BrO−(aq) Keq=2.8×10−9
The equilibrium reaction in 0.100M HBrO(aq) at equilibrium is represented by the equation above. Based on the magnitude of the equilibrium constant, which of the following correctly compares the equilibrium concentrations of substances involved in the reaction, and why?
answer choices
The equilibrium concentration of BrO− will be much smaller than the equilibrium concentration of H3O+, because H2O is the solvent and is present in the largest amount.
The equilibrium concentration of BrO− will be much smaller than the equilibrium concentration of HBrO, because Keq<<1
The equilibrium concentration of H3O+ will be much smaller than the equilibrium concentration of BrO−, because all the HBrO will react to produce BrO−
The equilibrium concentration of H3O+ will be much larger than the equilibrium concentration of HBrO, because Keq<<1.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 09:00
Which question could be best answered using the process of scientific inquiry? do different plates have different rock compositions? why did it take so long to develop the theory of plate tectonics? what are different cultural myths caused by plate tectonics? do plates move intentionally to cause volcanic eruptions?
Answers: 3

Chemistry, 23.06.2019 10:00
Abike ride event is 30 miles. a first aid tent is put at the 3/4 mark of the course. how many miles from the starting point is the first aid tent?
Answers: 1

Chemistry, 23.06.2019 15:30
Among these processes, which is the slowest chemical reaction? a. digesting food b. boiling an egg c. tarnishing of silver d. melting of a glacier
Answers: 2

You know the right answer?
HBrO(aq) + H2O(l) ⇄ H3O+(aq) + BrO−(aq) Keq=2.8×10−9
The equilibrium reaction in 0.100M...
The equilibrium reaction in 0.100M...
Questions

Mathematics, 03.06.2021 21:20


Social Studies, 03.06.2021 21:20

Social Studies, 03.06.2021 21:20

History, 03.06.2021 21:20

Computers and Technology, 03.06.2021 21:20

Mathematics, 03.06.2021 21:20

Mathematics, 03.06.2021 21:20

Mathematics, 03.06.2021 21:20

Mathematics, 03.06.2021 21:20


Social Studies, 03.06.2021 21:20



Chemistry, 03.06.2021 21:20

Mathematics, 03.06.2021 21:20

Health, 03.06.2021 21:20

Physics, 03.06.2021 21:20

English, 03.06.2021 21:20

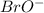 will be much smaller than the equilibrium concentration of
will be much smaller than the equilibrium concentration of 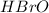 , because Keq<<1
, because Keq<<1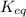
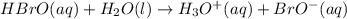
![K=\frac{[H_3O^+]\times [BrO^-]}{[HBrO]}](/tpl/images/0585/9548/ee59c.png)
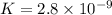
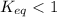 , That means the concentration of products is less as the reaction does not proceed much towards the forward direction.
, That means the concentration of products is less as the reaction does not proceed much towards the forward direction.

