Given the reaction:
HCl(g) + H2O(l) → H3O+(aq) + Cl−(aq)
Which reactant acted as a Brons...

Chemistry, 07.04.2020 05:32 kaitlksndj
Given the reaction:
HCl(g) + H2O(l) → H3O+(aq) + Cl−(aq)
Which reactant acted as a Bronsted-Lowry acid?
A) H2O(l), because it accepted protons
B) H2O(l), because it produced hydronium ions C) HCl(g), because it donated protons
D) HCl(g), because it reacted with chloride ions

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1

Chemistry, 23.06.2019 08:10
Time remaining 58: 10 an atom that has 84 protons and 86 neutrons undergoes a reaction. at the end of the reaction, it has 82 protons and 84 neutrons. what happened to the atom? it accepted radiation in a chemical reaction it donated neutrons to another atom in a chemical reaction it emitted an alpha particle in a nuclear reaction. it accepted protons in a nuclear reaction. mark this and retum save and exit next submit
Answers: 3
You know the right answer?
Questions







Biology, 10.12.2020 17:00



Biology, 10.12.2020 17:00





History, 10.12.2020 17:00


Mathematics, 10.12.2020 17:00



Law, 10.12.2020 17:00

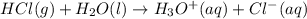
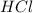 is loosing a proton, thus it is considered as an acid and after losing a proton, it forms
is loosing a proton, thus it is considered as an acid and after losing a proton, it forms 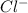 which is a conjugate base.
which is a conjugate base.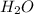 is gaining a proton, thus it is considered as a base and after gaining a proton, it forms
is gaining a proton, thus it is considered as a base and after gaining a proton, it forms 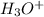 which is a conjugate acid.
which is a conjugate acid.


