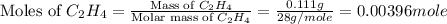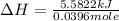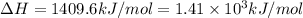Chemistry, 07.04.2020 15:12 kimberlylove387
A bomb calorimeter has a heat capacity of 2.47 kJ/K including the water. When a 0.111 g sample of ethylene (C 2H 4) was burned in this calorimeter, the temperature increased by 2.26 K. Calculate the energy of combustion for one mole of ethylene. a. -50.3 kJ/mol b. -1.41 x 103 kJ/mol c. -0.274 kJ/mol d. -624 kJ/mol e. -5.29 kJ/mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1


Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2

Chemistry, 23.06.2019 10:30
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
You know the right answer?
A bomb calorimeter has a heat capacity of 2.47 kJ/K including the water. When a 0.111 g sample of et...
Questions


Mathematics, 08.04.2020 02:51





History, 08.04.2020 02:51









Mathematics, 08.04.2020 02:51

Biology, 08.04.2020 02:51

Biology, 08.04.2020 02:51

Computers and Technology, 08.04.2020 02:51

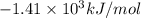
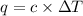
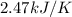
 = Change in temperature = 2.26 K
= Change in temperature = 2.26 K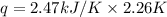
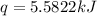
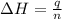
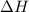 = energy of combustion for one mole of ethylene = ?
= energy of combustion for one mole of ethylene = ?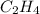 = 0.111 g
= 0.111 g