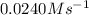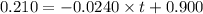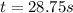Chemistry, 07.04.2020 15:59 angelricardoblp8kwg3
The rate constant for this zero‑order reaction is 0.0240 M ⋅ s − 1 0.0240 M·s−1 at 300 ∘ C. 300 ∘C. A ⟶ products A⟶products How long (in seconds) would it take for the concentration of A A to decrease from 0.900 M 0.900 M to 0.210 M?

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 08:00
Suppose a pair of chemical compounds a and b can react in two different ways: a + b -> c reaction 1 gives product c. a + b -> d reaction 2 gives product d. the following facts are known about the two reactions: . reaction 1 is endothermic and reaction 2 is exothermic. if a reaction vessel is charged (filled) with a and b , then at first d is produced faster than c. use these facts to sketch a qualitative reaction energy diagram for both reactions. note: because these sketches are only qualitative, the energies don? t have to be exact. they only have to have the right relationship to each other. for example, if one energy is less than another, that fact should be clear in your sketch.
Answers: 3


Chemistry, 23.06.2019 13:30
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 1

Chemistry, 24.06.2019 01:20
Type the correct answer in the box. express your answer to three significant figures. an empty water bottle is full of air at 15°c and standard pressure. the volume of the bottle is 0.500 liter. how many moles of air are in the bottle? the water bottle contains mole of air.
Answers: 2
You know the right answer?
The rate constant for this zero‑order reaction is 0.0240 M ⋅ s − 1 0.0240 M·s−1 at 300 ∘ C. 300 ∘C....
Questions


English, 08.03.2021 23:40

Mathematics, 08.03.2021 23:40

Mathematics, 08.03.2021 23:40

Mathematics, 08.03.2021 23:40


Social Studies, 08.03.2021 23:40

History, 08.03.2021 23:40




Chemistry, 08.03.2021 23:40

Advanced Placement (AP), 08.03.2021 23:40

Social Studies, 08.03.2021 23:40

Mathematics, 08.03.2021 23:40

Mathematics, 08.03.2021 23:40



Biology, 08.03.2021 23:40

Mathematics, 08.03.2021 23:40

![[A]=-kt+[A]_o](/tpl/images/0586/4094/d191d.png)
![[A]_o](/tpl/images/0586/4094/9caf5.png) = initial concentration = 0.900 M
= initial concentration = 0.900 M