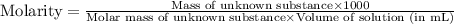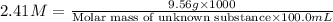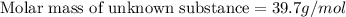Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
A chemical test has determined the concentration of a solution of an unknown substance to be 2.41 M....
Questions

History, 04.02.2020 14:53





Mathematics, 04.02.2020 14:53

Social Studies, 04.02.2020 14:53

Mathematics, 04.02.2020 14:53


Mathematics, 04.02.2020 14:53




History, 04.02.2020 14:53

Mathematics, 04.02.2020 14:53

Mathematics, 04.02.2020 14:53


English, 04.02.2020 14:53

Mathematics, 04.02.2020 14:53