Chemistry, 07.04.2020 16:51 maxi12312345
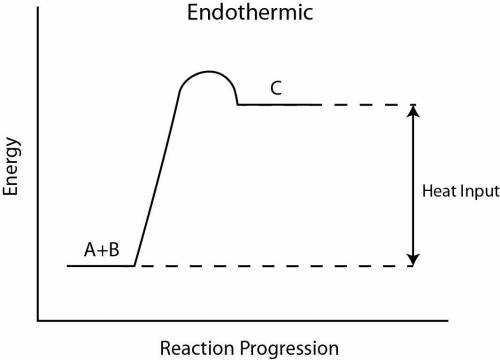
Two aqueous solutions are both at room temperature and are then mixed in a coffee cup calorimeter. The reaction causes the temperature of the resulting solution to fall below room temperature. Which of the following statements is TRUE? None of these statements are true. a. The products have a lower potential energy than the reactants. b. Energy is leaving the system during reaction. The reaction is exothermic. c. This type of experiment will provide data to calculate ΔErxn. d. The mixing is endothermic.
e. None of the above statements are true.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
Two aqueous solutions are both at room temperature and are then mixed in a coffee cup calorimeter. T...
Questions





Chemistry, 27.07.2019 00:20


Mathematics, 27.07.2019 00:20











Mathematics, 27.07.2019 00:20

History, 27.07.2019 00:20