
Chemistry, 07.04.2020 16:54 jagarcia2024
Calculate the [ OH − ] and the pH of a solution with an [ H + ] = 0.090 M at 25 °C . [ OH − ] = M pH = Calculate the [ H + ] and the pH of a solution with an [ OH − ] = 0.00098 M at 25 °C . [ H + ] = M pH = Calculate the [ H + ] and the [ OH − ] of a solution with a pH = 10.15 at 25 °C . [ H + ] = M [ OH − ] =

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1


Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3
You know the right answer?
Calculate the [ OH − ] and the pH of a solution with an [ H + ] = 0.090 M at 25 °C . [ OH − ] = M pH...
Questions






Chemistry, 19.11.2020 18:00




World Languages, 19.11.2020 18:00



Mathematics, 19.11.2020 18:00

English, 19.11.2020 18:00

Advanced Placement (AP), 19.11.2020 18:00

English, 19.11.2020 18:00


Social Studies, 19.11.2020 18:00


![[OH^-]=1.09\times 10^{-13}](/tpl/images/0586/5079/12eb0.png) and pH = 1.04
and pH = 1.04![[H^+]=1.02\times 10^{-11}](/tpl/images/0586/5079/f4659.png) and
and 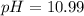
![[H^+]=7.08\times 10^{-11}](/tpl/images/0586/5079/77629.png) and
and ![[OH^-]=1.41\times 10^{-4}](/tpl/images/0586/5079/9fe1b.png)
![pH=-\log [H^+]](/tpl/images/0586/5079/37e81.png)
![pOH=-\log [OH^-]](/tpl/images/0586/5079/1fac1.png)
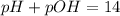
![[H^+]=0.090M](/tpl/images/0586/5079/6c0fa.png)
![pH=-\log [0.090]=1.04](/tpl/images/0586/5079/718ea.png)
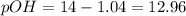
![12.96=-log[OH^-]](/tpl/images/0586/5079/9dc6e.png)
![[OH^-]=0.00098M](/tpl/images/0586/5079/a32b5.png)
![pOH=-\log [0.00098]=3.01](/tpl/images/0586/5079/735b6.png)
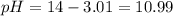
![10.99=-log[H^+]](/tpl/images/0586/5079/318f1.png)
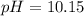
![10.15=-\log [H^+]](/tpl/images/0586/5079/097ad.png)
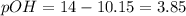
![3.85=-log[OH^-]](/tpl/images/0586/5079/b9fea.png)


