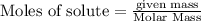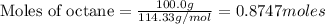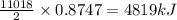Chemistry, 07.04.2020 17:02 cylertroutner
Using the following equation for the combustion of octane, calculate the heat associated with the combustion of 100.0 g of octane assuming complete combustion. The molar mass of octane is 114.33 g/mole. The molar mass of oxygen is 31.9988 g/mole.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 23.06.2019 10:00
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 1
You know the right answer?
Using the following equation for the combustion of octane, calculate the heat associated with the co...
Questions


Mathematics, 09.07.2019 22:30

Geography, 09.07.2019 22:30


Mathematics, 09.07.2019 22:30

Mathematics, 09.07.2019 22:30



Mathematics, 09.07.2019 22:30

Mathematics, 09.07.2019 22:30




Mathematics, 09.07.2019 22:30

History, 09.07.2019 22:30

Mathematics, 09.07.2019 22:30


Mathematics, 09.07.2019 22:30

English, 09.07.2019 22:30

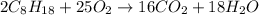 ΔH°rxn =-11018 kJ
ΔH°rxn =-11018 kJ