
Chemistry, 07.04.2020 16:59 shanasia76
A 25.0 g sample of an alloy was heated to 100.0 oC and dropped into a beaker containing 90 grams of water at 25.32 oC. The temperature of the water rose to a final value of 27.18 oC. Neglecting heat losses to the room and the heat capacity of the beaker itself, what is the specific heat of the alloy

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
A 25.0 g sample of an alloy was heated to 100.0 oC and dropped into a beaker containing 90 grams of...
Questions

Mathematics, 20.08.2019 05:30




Mathematics, 20.08.2019 05:30

Geography, 20.08.2019 05:30


World Languages, 20.08.2019 05:30

Biology, 20.08.2019 05:30



Mathematics, 20.08.2019 05:30

History, 20.08.2019 05:30


Mathematics, 20.08.2019 05:30

Mathematics, 20.08.2019 05:30


Social Studies, 20.08.2019 05:30

English, 20.08.2019 05:30

Biology, 20.08.2019 05:30

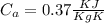
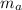 = 25 gm
= 25 gm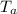 = 100°c = 373 K
= 100°c = 373 K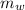 = 90 gm
= 90 gm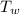 = 25.32 °c = 298.32 K
= 25.32 °c = 298.32 K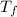 = 27.18 °c = 300.18 K
= 27.18 °c = 300.18 K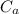 [
[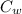 (
(

