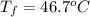Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2


Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
F 100. grams of liquid water at 100.°C and 200. grams of water at 20.0°C are mixed in an insulated c...
Questions

History, 31.01.2020 02:02



Mathematics, 31.01.2020 02:02

Advanced Placement (AP), 31.01.2020 02:02


Social Studies, 31.01.2020 02:02




Social Studies, 31.01.2020 02:02

Mathematics, 31.01.2020 02:02


History, 31.01.2020 02:02

History, 31.01.2020 02:02



English, 31.01.2020 02:02


Social Studies, 31.01.2020 02:02

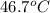
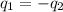
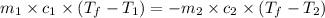
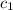 =
= 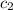 = specific heat of liquid water = Same
= specific heat of liquid water = Same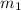 = mass of liquid water = 100 g
= mass of liquid water = 100 g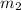 = mass of water = 200 g
= mass of water = 200 g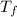 = final temperature of mixture = ?
= final temperature of mixture = ?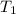 = initial temperature of liquid water =
= initial temperature of liquid water = 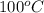
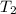 = initial temperature of water =
= initial temperature of water = 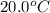
![(100g)\times (T_f-100.0)^oC=-[(200g)\times (T_f-20.0)^oC]](/tpl/images/0586/5833/2dfce.png)