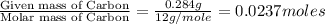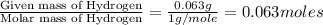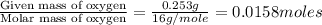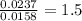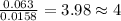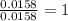Chemistry, 07.04.2020 17:26 Crtive6538
Combustion analysis of 0.600 g of an unknown compound containing carbon, hydrogen, and oxygen produced 1.043 g of CO2 and 0.5670 g of H2O. What is the empirical formula of the compound? Note because you are not able to enter subscripts enter the answer in the form: CxHyOz

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

You know the right answer?
Combustion analysis of 0.600 g of an unknown compound containing carbon, hydrogen, and oxygen produc...
Questions


Mathematics, 01.03.2021 17:40

Business, 01.03.2021 17:40



Arts, 01.03.2021 17:40

Mathematics, 01.03.2021 17:40

Mathematics, 01.03.2021 17:40

English, 01.03.2021 17:40


Mathematics, 01.03.2021 17:40


Chemistry, 01.03.2021 17:40


History, 01.03.2021 17:40

Mathematics, 01.03.2021 17:40

Social Studies, 01.03.2021 17:40

Mathematics, 01.03.2021 17:40


Mathematics, 01.03.2021 17:40

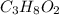
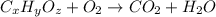
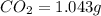
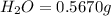
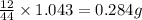 of carbon will be contained.
of carbon will be contained.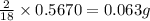 of hydrogen will be contained.
of hydrogen will be contained.