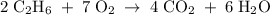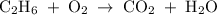Chemistry, 07.04.2020 17:26 jhashknkughb6759
When ethanol (C2H6O(aq)) is consumed, it reacts with oxygen gas (O2) in the body to produce gaseous carbon dioxide and liquid water. Enter the balanced chemical equation for the reaction

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
You know the right answer?
When ethanol (C2H6O(aq)) is consumed, it reacts with oxygen gas (O2) in the body to produce gaseous...
Questions

Mathematics, 29.09.2020 06:01


Biology, 29.09.2020 06:01


Physics, 29.09.2020 06:01

Mathematics, 29.09.2020 06:01





History, 29.09.2020 06:01

History, 29.09.2020 06:01

French, 29.09.2020 06:01



Mathematics, 29.09.2020 06:01

Mathematics, 29.09.2020 06:01


Mathematics, 29.09.2020 06:01