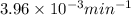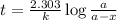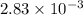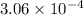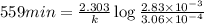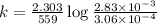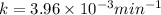Chemistry, 07.04.2020 19:03 hbkakabryce0p3fkoq
The gas phase decomposition of sulfuryl chloride at 600 K SO2Cl2(g)SO2(g) + Cl2(g) is first order in SO2Cl2. During one experiment it was found that when the initial concentration of SO2Cl2 was 2.83×10-3 M, the concentration of SO2Cl2 dropped to 3.06×10-4 M after 559 min had passed. Based on this experiment, the rate constant for the reaction is min-1.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
The isotonic saline solution described in part a is connected to an unknown solution via a semipermeable membrane, the unknown solution level drops. based on this information, what can be said about these two solutions?
Answers: 1


Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1
You know the right answer?
The gas phase decomposition of sulfuryl chloride at 600 K SO2Cl2(g)SO2(g) + Cl2(g) is first order in...
Questions

Mathematics, 30.03.2021 07:20

English, 30.03.2021 07:20


Mathematics, 30.03.2021 07:20

Biology, 30.03.2021 07:20

Geography, 30.03.2021 07:20


Mathematics, 30.03.2021 07:20

English, 30.03.2021 07:30



Mathematics, 30.03.2021 07:30

Spanish, 30.03.2021 07:30

Chemistry, 30.03.2021 07:30

Mathematics, 30.03.2021 07:30


Chemistry, 30.03.2021 07:30

Mathematics, 30.03.2021 07:30