

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1
You know the right answer?
A sample of argon gas, Ar(g). is placed in a 3.80 L container at 320 K. The gas pressure is 0.496 at...
Questions

Mathematics, 04.06.2021 04:50



Social Studies, 04.06.2021 04:50

Chemistry, 04.06.2021 04:50



Mathematics, 04.06.2021 04:50

History, 04.06.2021 04:50

English, 04.06.2021 04:50


Geography, 04.06.2021 04:50




Social Studies, 04.06.2021 04:50


Mathematics, 04.06.2021 04:50

Biology, 04.06.2021 04:50

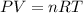 .......(1)
.......(1)


