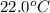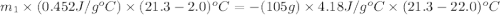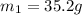Chemistry, 07.04.2020 19:02 precioushayhay
105 mL of H2O is initially at room temperature (22.0∘C). A chilled steel rod at 2.0∘C is placed in the water. If the final temperature of the system is 21.3 ∘C, what is the mass of the steel bar? Specific heat of water = 4.18 J/g⋅∘C Specific heat of steel = 0.452 J/g⋅∘C

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1


Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
105 mL of H2O is initially at room temperature (22.0∘C). A chilled steel rod at 2.0∘C is placed in t...
Questions


Physics, 09.09.2019 20:30

English, 09.09.2019 20:30

Mathematics, 09.09.2019 20:30



Computers and Technology, 09.09.2019 20:30



Computers and Technology, 09.09.2019 20:30






History, 09.09.2019 20:30

Mathematics, 09.09.2019 20:30


Mathematics, 09.09.2019 20:30

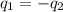
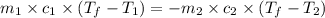
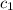 = specific heat of steel =
= specific heat of steel = 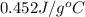
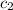 = specific heat of water =
= specific heat of water = 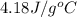
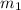 = mass of steel rod = ?
= mass of steel rod = ?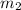 = mass of water =
= mass of water = 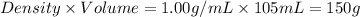
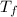 = final temperature of mixture =
= final temperature of mixture = 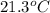
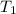 = initial temperature of steel =
= initial temperature of steel = 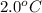
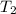 = initial temperature of water =
= initial temperature of water =