
Chemistry, 07.04.2020 19:41 BaileyRyan8320
A sample of pure NH4HS is placed in a sealed 2.0-L container and heated to 550 K at which the equilibrium constant is 3.5 x 10-3. Once the reaction reaches equilibrium, what mass of NH3 is present in the container

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
A sample of pure NH4HS is placed in a sealed 2.0-L container and heated to 550 K at which the equili...
Questions

Mathematics, 24.11.2020 19:50


Biology, 24.11.2020 19:50


English, 24.11.2020 19:50







Mathematics, 24.11.2020 19:50


SAT, 24.11.2020 19:50


History, 24.11.2020 19:50

History, 24.11.2020 19:50



Biology, 24.11.2020 19:50

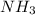 in the container is 2.074 gram
in the container is 2.074 gram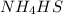
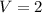 lit
lit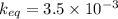
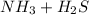
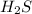 is formed.
is formed.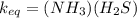
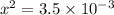
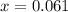 M
M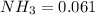
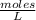
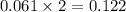 moles.
moles.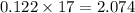 g
g

