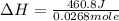Chemistry, 07.04.2020 20:35 quiyansimmons15
When a 2.00 g sample of KCl is dissolved in water in a calorimeter that has a total heat capacity of 1.28 kJ ⋅ K − 1 , the temperature decreases by 0.360 K . Calculate the molar heat of solution of KCl .

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1
You know the right answer?
When a 2.00 g sample of KCl is dissolved in water in a calorimeter that has a total heat capacity of...
Questions

Mathematics, 05.11.2020 21:20

English, 05.11.2020 21:20

Mathematics, 05.11.2020 21:20

Mathematics, 05.11.2020 21:20



Mathematics, 05.11.2020 21:20


Mathematics, 05.11.2020 21:20

Advanced Placement (AP), 05.11.2020 21:20

English, 05.11.2020 21:20


History, 05.11.2020 21:20



Mathematics, 05.11.2020 21:20


Mathematics, 05.11.2020 21:20

Mathematics, 05.11.2020 21:20

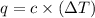
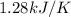
 = change in temperature = 0.360 K
= change in temperature = 0.360 K


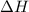 = enthalpy change = ?
= enthalpy change = ?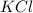 = 2.00 g
= 2.00 g