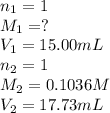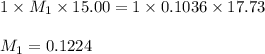Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
7. A student titrated a 15.00-mL sample of a solution containing a weak, monoprotic acid with NaOH....
Questions

Physics, 20.05.2020 22:57

Mathematics, 20.05.2020 22:57


Mathematics, 20.05.2020 22:57





Geography, 20.05.2020 22:57


Mathematics, 20.05.2020 22:57

Biology, 20.05.2020 22:57

Mathematics, 20.05.2020 22:57


History, 20.05.2020 22:57


Mathematics, 20.05.2020 22:57

Mathematics, 20.05.2020 22:57

Mathematics, 20.05.2020 22:57


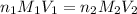
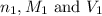 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 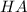
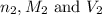 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.