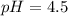Chemistry, 07.04.2020 20:01 lmcginnis2003
Calculate the pH of a solution prepared by dissolving 0.15 mol benzoic acid (C7H5O2H) and 0.30 mol of sodium benzoate (Na C7H5O2) in water sufficient to yield 1.00 L of solution. The Ka of benzoic acid is 6.50 x 10-5

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
Calculate the pH of a solution prepared by dissolving 0.15 mol benzoic acid (C7H5O2H) and 0.30 mol o...
Questions

Chemistry, 12.11.2020 19:20


Arts, 12.11.2020 19:20

History, 12.11.2020 19:20

History, 12.11.2020 19:20

Mathematics, 12.11.2020 19:20


Biology, 12.11.2020 19:20

Mathematics, 12.11.2020 19:20




Mathematics, 12.11.2020 19:20


History, 12.11.2020 19:20



Mathematics, 12.11.2020 19:20

English, 12.11.2020 19:20

Mathematics, 12.11.2020 19:20

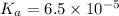
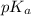 .
.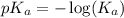
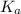 in this expression, we get:
in this expression, we get: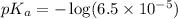
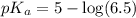
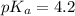
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0587/0629/e961a.png)
![pH=4.2+\log [\frac{(\frac{0.30}{1.00L})}{(\frac{0.15}{1.00L})}]](/tpl/images/0587/0629/17ffa.png)