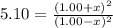Chemistry, 07.04.2020 21:10 kenishawilkinsoy4mgw
Carbon Monoxide reacts with steam to produce carbon dioxide and hydrogen. At 700K, the equilibrium constant is 5.10. Calculate the equilibrium concentrations of all species if 1.00mol of each component is mixed in a 1.00L flask.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3


Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
Carbon Monoxide reacts with steam to produce carbon dioxide and hydrogen. At 700K, the equilibrium c...
Questions

English, 08.03.2021 23:50







Mathematics, 08.03.2021 23:50

SAT, 08.03.2021 23:50

Social Studies, 08.03.2021 23:50

Mathematics, 08.03.2021 23:50


Chemistry, 08.03.2021 23:50

Mathematics, 08.03.2021 23:50

Mathematics, 08.03.2021 23:50

Mathematics, 08.03.2021 23:50

Mathematics, 08.03.2021 23:50

Mathematics, 08.03.2021 23:50


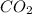 at equilibrium= 1.386 M
at equilibrium= 1.386 M 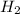 at equilibrium = 1.386 M
at equilibrium = 1.386 M 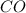 at equilibrium = 0.614 M
at equilibrium = 0.614 M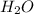 at equilibrium= 0.614 M
at equilibrium= 0.614 M 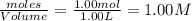
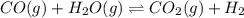
![K_c=\frac{[CO_2]\times [H_2]}{[CO]\times [H_2O]}](/tpl/images/0587/3047/a0f86.png)