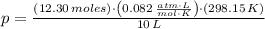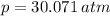Chemistry, 07.04.2020 21:13 ondreabyes225pcr83r
At 25.0 ° C, a 10.00 L vessel is filled with 5.25 moles of Gas A and 7.05 moles of Gas B. What is the total pressure?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

You know the right answer?
At 25.0 ° C, a 10.00 L vessel is filled with 5.25 moles of Gas A and 7.05 moles of Gas B. What is th...
Questions


English, 20.09.2021 14:30

Mathematics, 20.09.2021 14:30



Geography, 20.09.2021 14:30

Mathematics, 20.09.2021 14:30

English, 20.09.2021 14:30


Chemistry, 20.09.2021 14:30

English, 20.09.2021 14:30

English, 20.09.2021 14:30


Physics, 20.09.2021 14:30

Mathematics, 20.09.2021 14:30


Mathematics, 20.09.2021 14:30

Mathematics, 20.09.2021 14:30

Biology, 20.09.2021 14:30

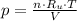 (1)
(1)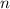 - Total amount of moles, in moles.
- Total amount of moles, in moles.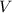 - Volume of the vessel, in liters.
- Volume of the vessel, in liters.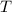 - Temperature inside the vessel, in Kelvin.
- Temperature inside the vessel, in Kelvin.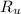 - Ideal gas constant, in atmosphere-liters per mole-Kelvin.
- Ideal gas constant, in atmosphere-liters per mole-Kelvin.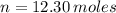 ,
, 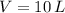 ,
, 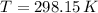 and
and 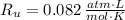 , then the total pressure is:
, then the total pressure is: