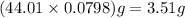Gaseous methane (CH4) reacts with gaseous oxygen (O2) gas to produce gaseous carbon dioxide (CO2) and gaseous water (H2O). What is the theoretical yield of carbon dioxide formed from the reaction of 1.28 g of methane and 10.1 g of oxygen gas? Be sure your answer has the correct number of significant digits in it.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

You know the right answer?
Gaseous methane (CH4) reacts with gaseous oxygen (O2) gas to produce gaseous carbon dioxide (CO2) an...
Questions


Mathematics, 12.03.2021 22:50

Chemistry, 12.03.2021 22:50



Computers and Technology, 12.03.2021 22:50

Mathematics, 12.03.2021 22:50


Mathematics, 12.03.2021 22:50


Mathematics, 12.03.2021 22:50



Mathematics, 12.03.2021 22:50

Social Studies, 12.03.2021 22:50


Mathematics, 12.03.2021 22:50



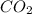 is 3.51 g.
is 3.51 g.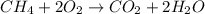
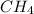 = 16.04 g/mol
= 16.04 g/mol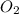 = 32.00 g/mol
= 32.00 g/mol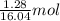 of
of 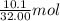 of
of